BACKGROUND
In a Phase I study (NCT04000165, Xu et al, 2022), we showed that mitapivat, an investigational oral allosteric activator of pyruvate kinase (PK), demonstrated an acceptable safety and tolerability profile at multiple ascending dose levels in patients (pts) with sickle cell disease (SCD). We also established proof-of-concept for activating PK as a viable therapeutic approach; mitapivat decreased 2,3- diphosphoglycerate (2,3-DPG) and increased adenosine triphosphate (ATP) levels, improved hematologic parameters, and reduced sickling in pts with SCD (HbSS). In this extension study (NCT04610866), we evaluated the safety, tolerability and efficacy of mitapivat as a long-term maintenance therapy for pts with SCD.
METHODS
We enrolled 15 adult pts (age >18 years) with confirmed SCD (HbSS) and baseline hemoglobin (Hb) 7.1 - 10.5 g/dL, with no recent blood transfusions, erythropoietin therapy, or changes in SCD-specific therapies including hydroxyurea (HU) and L-glutamine. Thirteen pts had participated in the Phase 1 study and 2 pts were mitapivat naïve. All pts started mitapivat at 50 mg twice daily (BID), escalating after 4 weeks (wks) to 100 mg BID, unless a rapid Hb rise was observed (increase of ≥2 g/dL), the maximum Hb level of 12.5 g/dL had been reached, or per PI discretion. Patients continued on this maintenance dose of mitapivat through the end of the study. Dose reductions were performed at any time during the extension phase for safety and tolerability. After completion of the 24-wk core period, pts had an option to continue on mitapivat treatment in the extension period for up to 6 years. Study visits were conducted every 2 wks (x2), 4 wks (x2), and then every 12 wks for first 2 years (yrs) and will continue every 6 months for the remaining 4 yrs. The extension period of the study is ongoing; here we report data for up to 2 years (data cutoff March 23, 2023). Any and all vaso-occlusive crises (VOCs) that occurred while on study, regardless of attribution, were considered adverse events.
RESULTS
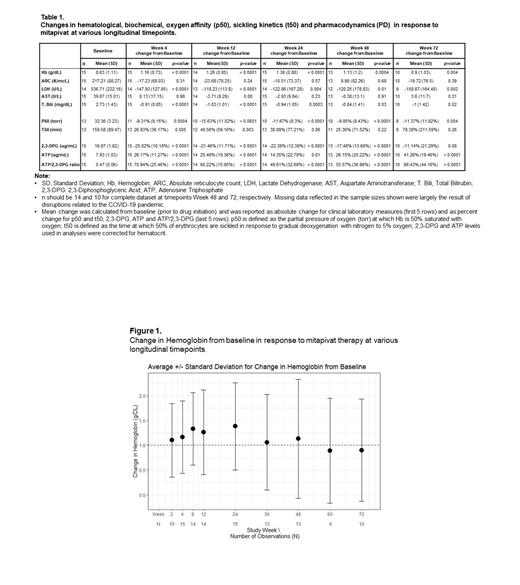
Of the 15 pts who completed the core period, 14 entered the extension study with 1 pt on 5 mg BID, 1 pt on 50 mg BID, 3 alternating between 50 and 100 mg BID, and the rest on 100 mg BID. Mean age of the 15 pts was 39 yrs (range: 25-57 yrs); 10 were male, and 11 were on HU. As of the cut-off date, median duration of mitapivat treatment for pts who entered the extension period was 84 wks (range: 48-108 wks), 1 pt discontinued after 2 yrs (pt decision) and 10 pts received 72 wks or more of treatment (Table 1) with a total of 1080 pt-wks of drug exposure thus far.
Mitapivat was generally well tolerated; no treatment emergent adverse events (TEAEs) resulted in discontinuation of study drug or death during the extension period. The most common TEAEs (reported in 5 or more pts) were VOCs (n=8), estrone decrease (n=7), testosterone increase (n=6) and cough (n = 5); changes in laboratory values were not clinically significant. The most common serious TEAEs (reported in 2 or more pts) were VOCs (n=8) and lung infection (n = 2). Two pts experienced VOCs that were assessed as possibly drug-related (1 during drug escalation, and 1 during drug taper). All VOCs occurred in the setting of known VOC triggers. Three pts required dose reduction, 1 each secondary to pruritis, bloating and insomnia, respectively. In the latter 2 cases, the doses were subsequently increased to 100 mg BID, as TEAEs resolved. Other serious TEAEs were not considered related to study drug.
The mean Hb at 24 wks increased significantly from baseline (mean: 1.38 g/dL, SD: 0.88 g/dL; p<0.0001), a result generally upheld at other follow-up periods (Fig. 1). 14/15 (93%) of the pts achieved a Hb response, defined as a ≥ 1 g/dL increase in Hb at any timepoint within the core period, compared to baseline. Improvements in Hb achieved in the core period were accompanied by improvements in markers of hemolysis, oxygen affinity (p50), sickling kinetics (t50), decreases in 2,3-DPG and increases in ATP levels, and were sustained in the extension study (Fig. 1 and 2). Concurrently, there were also improvements in red cell deformability (LORRCA) (Lundt et al, 2022) and trends in improvements in cardiopulmonary status (not shown).
CONCLUSION
Long-term use of mitapivat is safe and well tolerated in pts with SCD with evidence of sustained long-term improvements in Hb, hemolytic and sickling kinetics. Mitapivat offers a novel disease-modifying approach.
OffLabel Disclosure:
Xu:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios Pharmaceuticals, Inc.: Other: US national principal investigator for the Phase 1 clinical trial pf AG-946 in patients with sickle cell disease.. Yates:Agios Pharmaceuticals Inc.: Current Employment, Current equity holder in publicly-traded company. Wind-Rotolo:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company.
Mitapivat as an anti-sicking therapy in patients with sickle cell disease

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal